unlocking the secrets of biomacromolecules
Cryo-EM
Single Particle Analysis
Leveraging our advanced cryo-EM facility and expert team, Shuimu BioSciences excels in Single Particle Analysis, delivering precise high-resolution structures for drug formulation and gene therapy. With over 2000 SPA projects completed and resolutions reaching down to 1.4Å, we lead the way in advancing structural biology and drug discovery.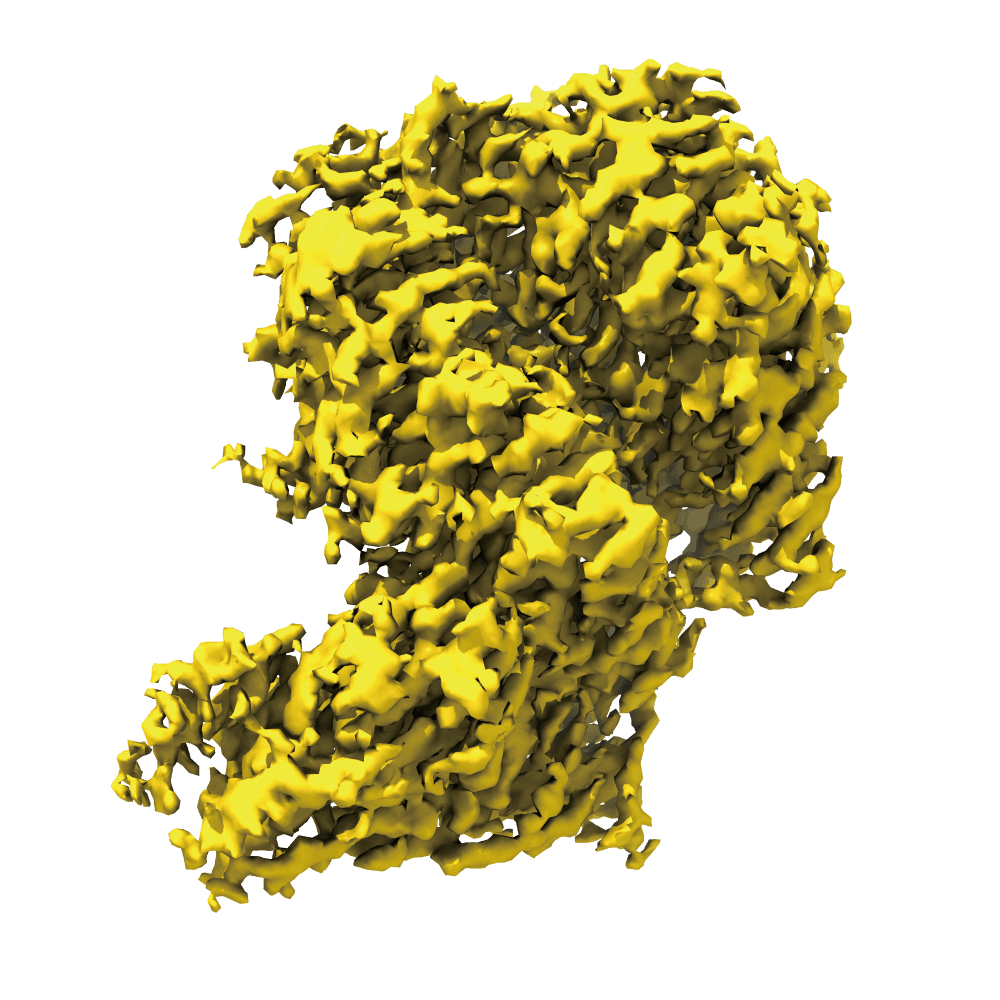
application
Cryo-EM SPA Application
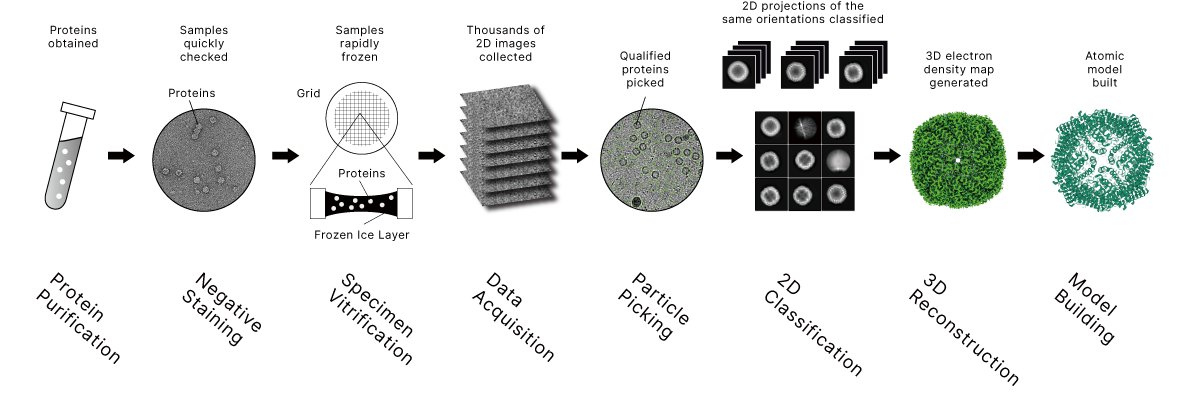
Workflow
Cryo-EM SPA Workflow
Why Choose Cryo-EM SPA?
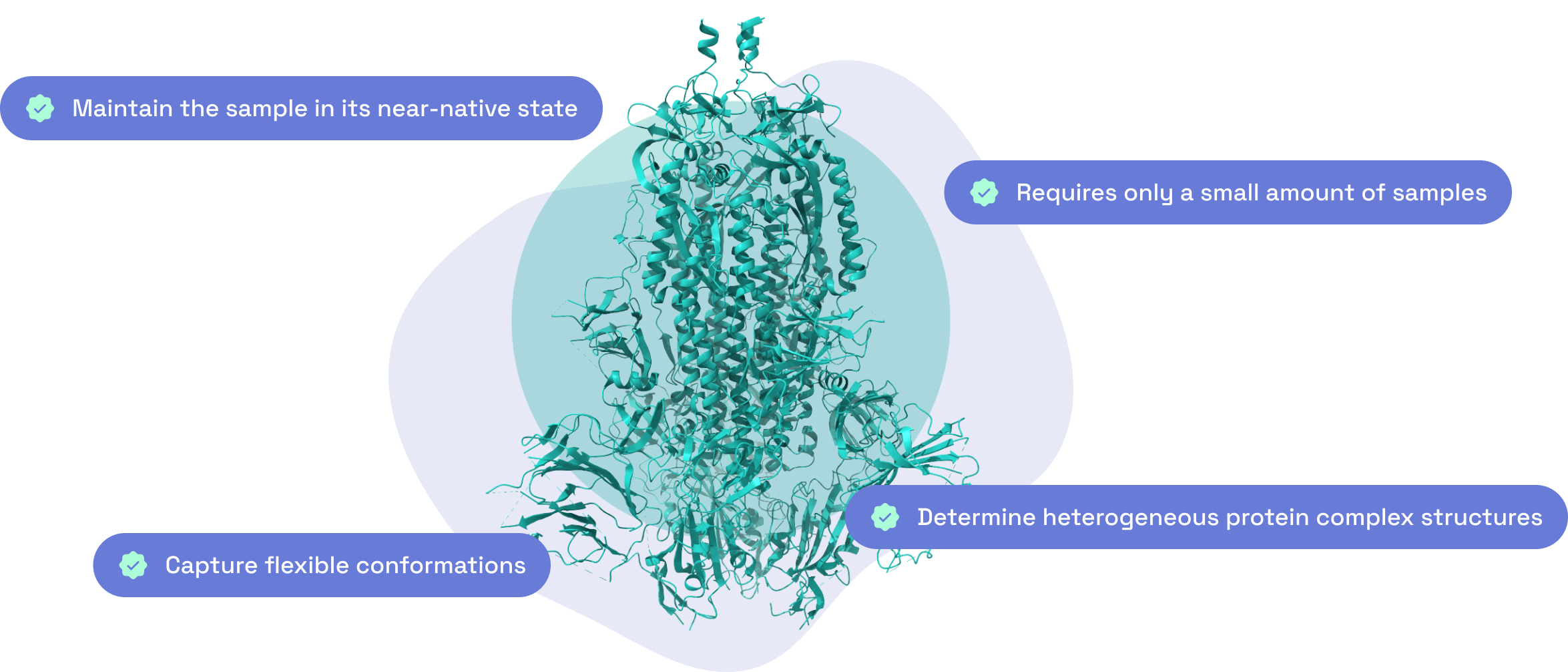
Why choose us?
Shuimu Cryo-EM SPA Advantages
Advanced Cryo-EM Facility
The highest specs microscopes & advanced computing platform for high-quality structure determinationCutting Edge Scientist Team
A team of PhD scientists from the top institutions, expertised in structural biology, protein science and computationProfound Experience
2000+ cryo-EM projects experience, from membrane proteins to antigen-antibody complexesResolution Pursuit
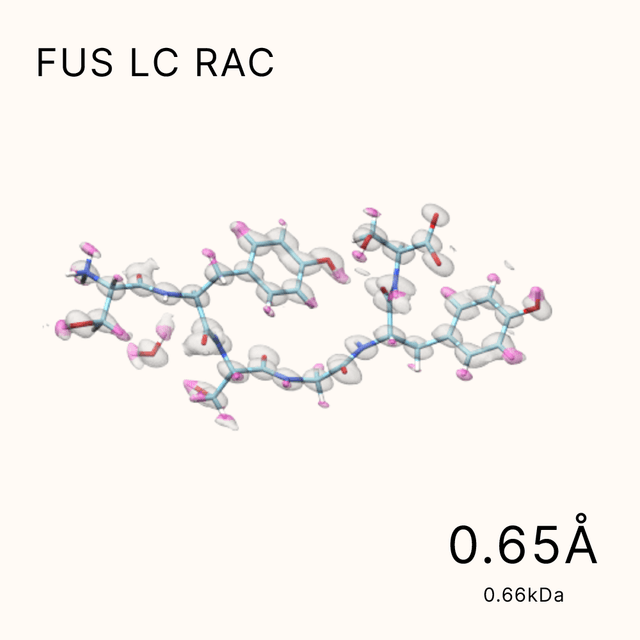
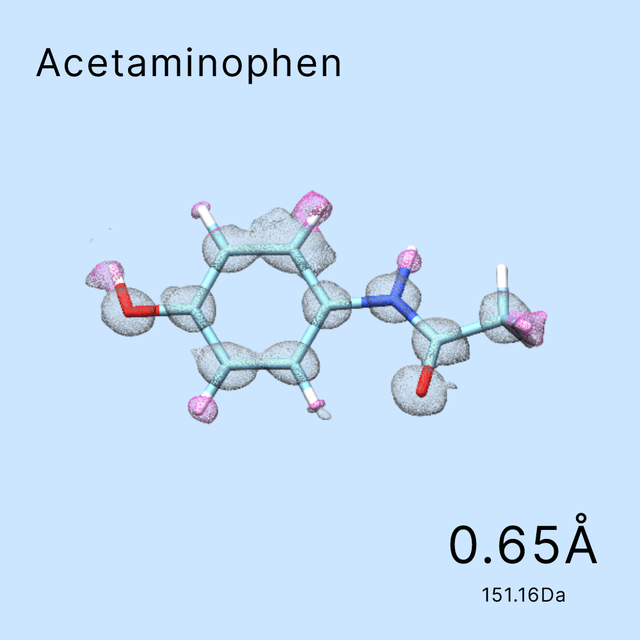
1200+ protein structures determined at resolution better than 3.5Å: the best at 1.4Å and the smallest being 51kDAWhat cases have we done?
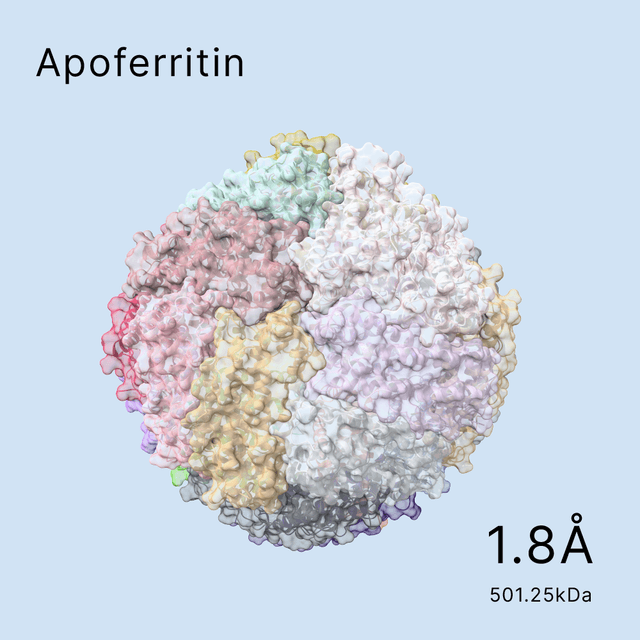
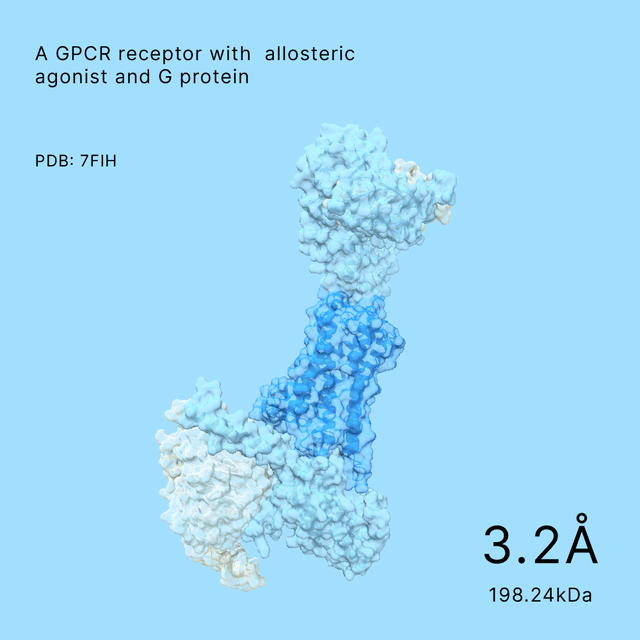
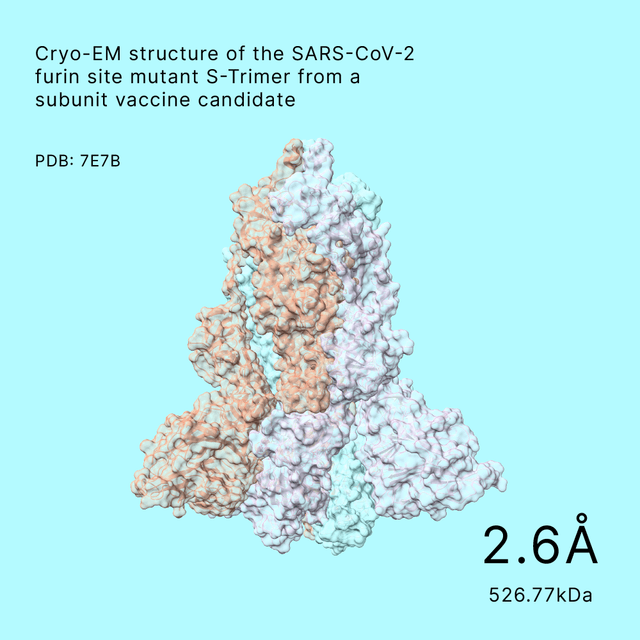
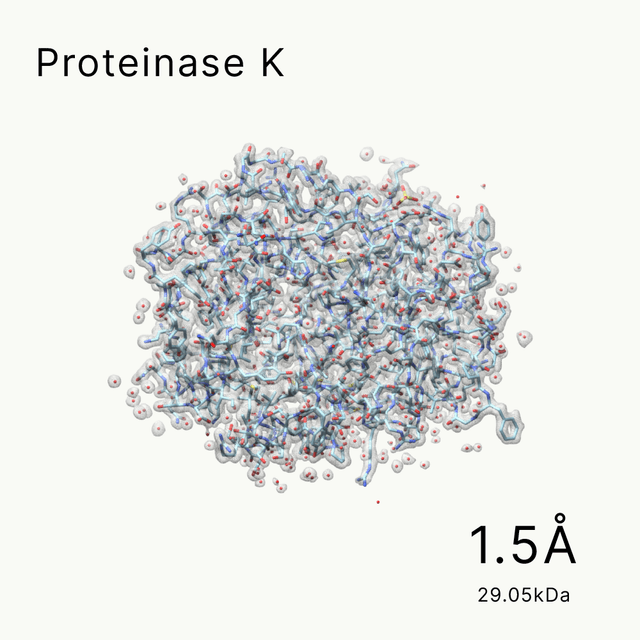
Structures Solved by Shuimu
Our platform has delivered high-quality cryo-EM and microED structures to over 400 clients since 2017.key milestones
Important Events
TOOLKIT
Shuimu Technology
We've developed unique tool kits to excel in Cryo-EM structure determination.See Unseeable.
Drug Undruggable.
Founded in 2017, Shuimu BioSciences aims to bring the power of cryo-EM to innovative therapeutics developers.Contacts
1 Broadway 5th floor, Cambridge, MA 02142, United States+1 (650) 680 9383
Hi@shuimubio.com
Copyright © 2025 Shuimu Biosciences Ltd.
京ICP备2020035593号